Did you get it?Learn the basics about plant oils and how they are extracted At Fuse School, teachers and animators come together to make fun & easytounderstand videos inSolid/liquid, liquid/liquid, and acid/base extraction Solid liquid involves the removal of a substance from a natural product or solid mixture (ie hot water and tea/coffee)

Difference Between Extraction And Isolation Compare The Difference Between Similar Terms
Types of extraction chemistry
Types of extraction chemistry-The extraction methods discussed in this chapter are most applicable to organic solutes that are considered nonvolatile and semivolatile However, it is possible to extend these techniques to more volatile chemicals as long as careful consideration of the tendency of the solute to volatilize is made throughout the extraction process 212Busque trabalhos relacionados a Types of extraction chemistry ou contrate no maior mercado de freelancers do mundo com mais de de trabalhos Cadastre




Extraction Liquid Liquid
Solvent Extraction Solvent extraction is a common form of chemical extraction using organic solvent as the extractant It is commonly used in combination with other technologies, such as solidification/stabilization, incineration, or soil washing, depending upon sitespecific conditions676 Radiochemistry and Nuclear Chemistry and 2 is the phase volume ratio vorg/vaqThe percentage solute extracted, denoted E(%), is equal to 100 R1The fraction of nonextracted solute left in the organic phase is n1, and R1 n1 = 1, n1 = 1/(P 1) (A5) Assume that one wants to separate uranium from the fission product lanthanum using the Extraction is the first step to separate the desired natural products from the raw materials Extraction methods include solvent extraction, distillation method, pressing and sublimation according to the extraction principle Solvent extraction is
Aqueous Two Phase Extraction They are used for organicwater solvent system They are excellent for the extraction of enzymes and proteins Batchwise Single Stage Extraction It is used in smallscale chemical industries The extraction is carried out in a simple separator funnel Multistage Countercurrent Continuous ProcessThe concept of liquid liquid extraction?Extraction in chemistry is a separation process consisting in the separation of a substance from a matrix Common examples include liquidliquid extraction, and solid phase extraction The distribution of a solute between two phases is an equilibrium condition described by
Search for jobs related to Types of extraction chemistry or hire on the world's largest freelancing marketplace with m jobs It's free to sign up and bid on jobsAcidbase extractions are used to either directly isolate a desired acidic or basic compound or to remove acidic or basic impurities Extraction, along with recrystallization and distillation, is one of the most important separation and purification techniques in organic chemistry An ore is any naturallyoccurring source of a metal that you can economically extract the metal from Aluminum, for example, is the most common metal in the Earth's crust, occurring in all sorts of minerals However, it isn't economically worthwhile to extract it from most of these minerals Instead, the usual ore of aluminum is bauxite which



Analytical Methods Of Isolation And Identification Intechopen




Extraction In The Organic Chemistry Teaching Labs
THC extraction methods are utilized to separate parts of the cannabis plant for various products The cannabis plant can be used for its plant material or extracts that have various beneficial chemicals Extraction is used specifically to isolate and get the desired compounds Cannabis has approximately 113 cannabinoids that include CBD Types Of Extraction Chemistry Solid Phase Extraction Technique A Trends Opportunities And Extraction (album), an album by guitarist greg howe Iron and aluminium are extracted from their ores in different ways because the metals have different reactivities Botanicals and herbal preparations for medicinal usage contain various types of bioactive compounds The focus of this paper is on the analytical methodologies, which include the extraction, isolation and characterization of active ingredients in botanicals and



1




Valorization Of Apple Pomace By Extraction Of Valuable Compounds Perussello 17 Comprehensive Reviews In Food Science And Food Safety Wiley Online Library
Types of Chromatography The different types of chromatographic techniques are on the basis of the mobile and stationary phases used Chromatography may be regarded as an analytical technique employed for the purification and separation of organic and inorganic substances The effects of grinding (mediumcoarse) and extraction time (14–22 h) on the physicochemical and sensorial properties of cold brew coffee produced using two types of Colombian specialty coffeesExtraction in chemistry is a separation process consisting in the separation of a substance from a matrix It includes Liquidliquid extraction, and Solid phase extraction The distribution of a solute between two phases is an equilibrium condition




Advantages And Disadvantages Of The Two Extraction Methods Download Table



A Review Of Sustainable And Intensified Techniques For Extraction Of Food And Natural Products Green Chemistry Rsc Publishing Doi 10 1039 C9gcg
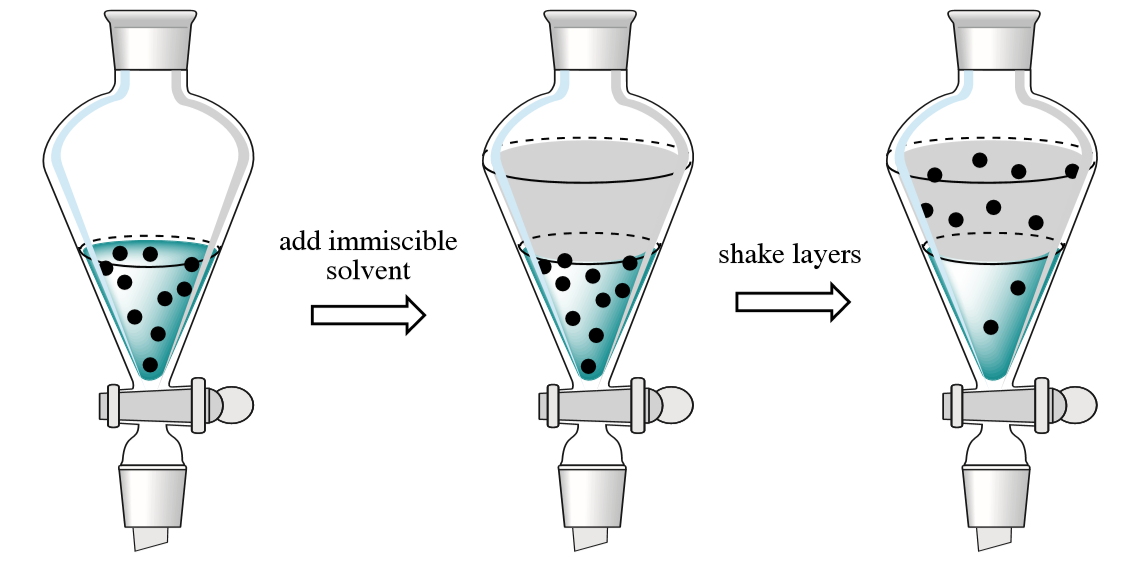
The three most common types of extractions are liquid/liquid, liquid/solid, and acid/base (also known as a chemically active extraction) The coffee and tea examples are both of the liquid/solid type in which a compound (caffeine) is isolated from a solid mixture by using a liquid extractionMethod to separate compounds or metal complexes Solvent extraction Liquid–liquid extraction ( LLE ), also known as solvent extraction and partitioning, is a method to separate compounds or metal complexes, based on their relative solubilities in two different immiscible liquids, usually water (polar) and an organic solvent (nonpolar)Solvent extraction is a common technique utilized for both industrial applications and in the laboratory The technique is successfully applied as a sample preparation procedure for chromatography



Physical Chemistry Theory



Separating Components Of A Mixture By Extraction Youtube
42 CounterCurrent ExtractionCounterCurrent Extraction A liquidliquid extraction process in which the solvent and the process stream in contact with each other flow in opposite directions Screw extractors and carousel extractors are the two type of equipments used for CounterCurrent ExtractionCommon extraction techniques for solid matrices include Soxhlet extraction, sonication extraction, supercritical fluid extraction (SFE), microwaveassisted extraction (MAE), and acceleratedsolvent extraction (ASE)Extraction Theory and General Procedure (Adapted from Mohrig, pp 5764, 7277) Extraction is a very common laboratory procedure used when isolating or purifying a product Organic chemistry employs solidliquid, liquidliquid, and acidbase extractions




Describe The Batch Extraction In The Solvent Extraction Method Solvent Extracton Analytical Youtube




Gcse 1 Introduction To The Extraction Of Metals Method Related To The Activity Of Reactivity Series Of Metals Igcse O Level Ks4 Science Chemistry Revision Notes Revising
There are three types of extraction; She has taught high school, AP chemistry for 2 years and is teaching undergraduate college chemistry for 3 years This lesson will define solvent extraction and discuss and explain theMetals with an intermediate reactivity, such as iron and zinc, are chemically extracted from minerals by a process known as carbon reduction Carbon reduction is a type of redox reaction, where carbon is used to displace a metal from a compound The crushed ore is mixed with carbon and limestone, then heated to a molten state (smelted) in a
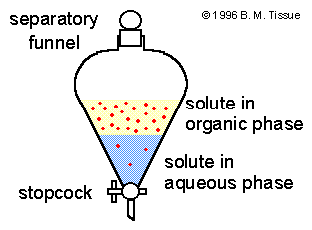



Definition Of Extraction Chemistry Dictionary



The Complete Guide To Hydrocarbon Extraction New In 19
Solvent extraction, principle, technique, types of extraction techniques with figure, Factors affecting extraction efficiency,and applications Liquid liquid extraction is a technique in which a solution (usually aqueous) is brought in contact with a second solvent (usually organic), essentiaExamples of solvent extraction are the extraction of uranium and plutonium salts from solution in nitric acid in nuclear fuel reprocessing using kerosene as solvent, and the extraction of benzene from reformed naphtha using sulfolane as solvent Protein extraction can be a difficult process, This rigorously tested kit is prepared with the highest standard of pure chemicals since extracting different types of proteins needs unique levels of processing CNMCS Kit Extracting from Tissues



Methods Of Extraction Refining And Concentration Of Fish Oil As A Source Of Omega 3 Fatty Acids



Methods Of Extraction Refining And Concentration Of Fish Oil As A Source Of Omega 3 Fatty Acids
Extraction in chemistry is a separation process consisting in the separation of a substance from a matrix It includes Liquidliquid extraction, and Solid phase extraction The distribution of a solute between two phases is an equilibrium condition described by partition theory This is based on exactly how the analyte move from the water into an organic layer Extraction processes 1 1 EXTRACTION PROCESSES 2 INTRODUCTION & DEFINITIONS 2 Extraction may be defined as the removal of soluble constituents from a solid or liquid or semisolid with means of suitable solvent It may be defined as the treatment of the plant or animal tissues with appropriate solvent, which would dissolve the medicinally active constituents ExtractionExtraction in chemistry is a separation process consisting in the separation of a substance from a matrixCommon examples include liquidliquid extraction, and solid phase extractionThe distribution of a solute between two phases is an equilibrium condition described by partition theory This is based on exactly how the analyte moves from the initial solvent into the extracting




Chemical Data Extraction Chemaxon
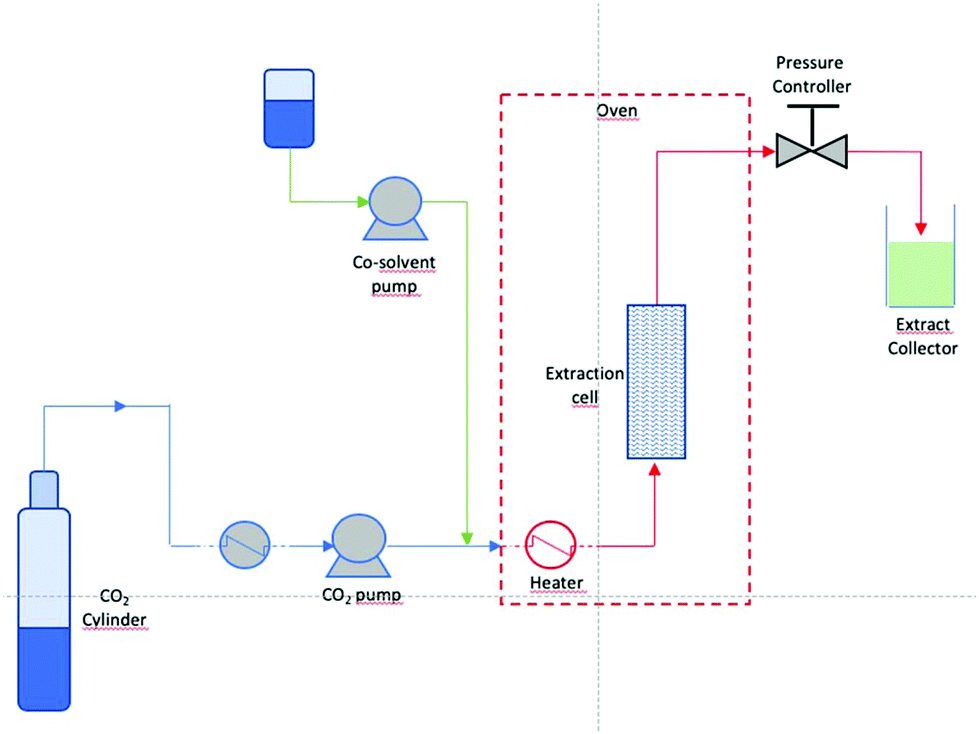



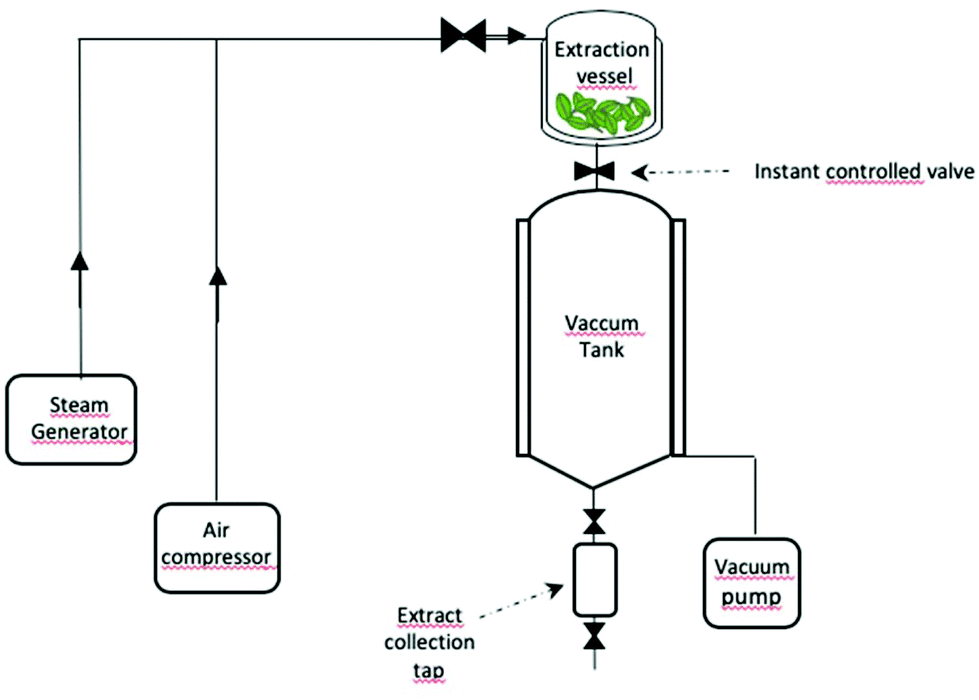
A Review Of Sustainable And Intensified Techniques For Extraction Of Food And Natural Products Green Chemistry Rsc Publishing Doi 10 1039 C9gcg
Metal Method of extraction Potassium Electrolysis Sodium Electrolysis Calcium Electrolysis Magnesium Electrolysis Aluminium Electrolysis Zinc Heat with carbon or carbon monoxide Iron Heat with carbon or carbon monoxide Lead Heat with carbon or carbon monoxide Copper Roasting in air Silver Occur naturally Gold Occur naturally Examples of the different methods of extraction 5 Types of Extraction 1 Electrolysis Electrolysis is a separation technique in which a direct current passes through an ionic substance in 2 Propolis Propolis is a mixture containing resin collected by bees from tree buds and plant resins Bees use propolis 3 In the chemistry lab, essential oils are often extracted from their source using solvents, and analyzed using gas chromatography or spectroscopy Transferring Compounds From Layers Another method for extracting essential oils from fragrant plant materials is through steam distillation (Figure 44b)




Difference Between Extraction And Isolation Compare The Difference Between Similar Terms




Difference Between Extraction And Isolation Compare The Difference Between Similar Terms
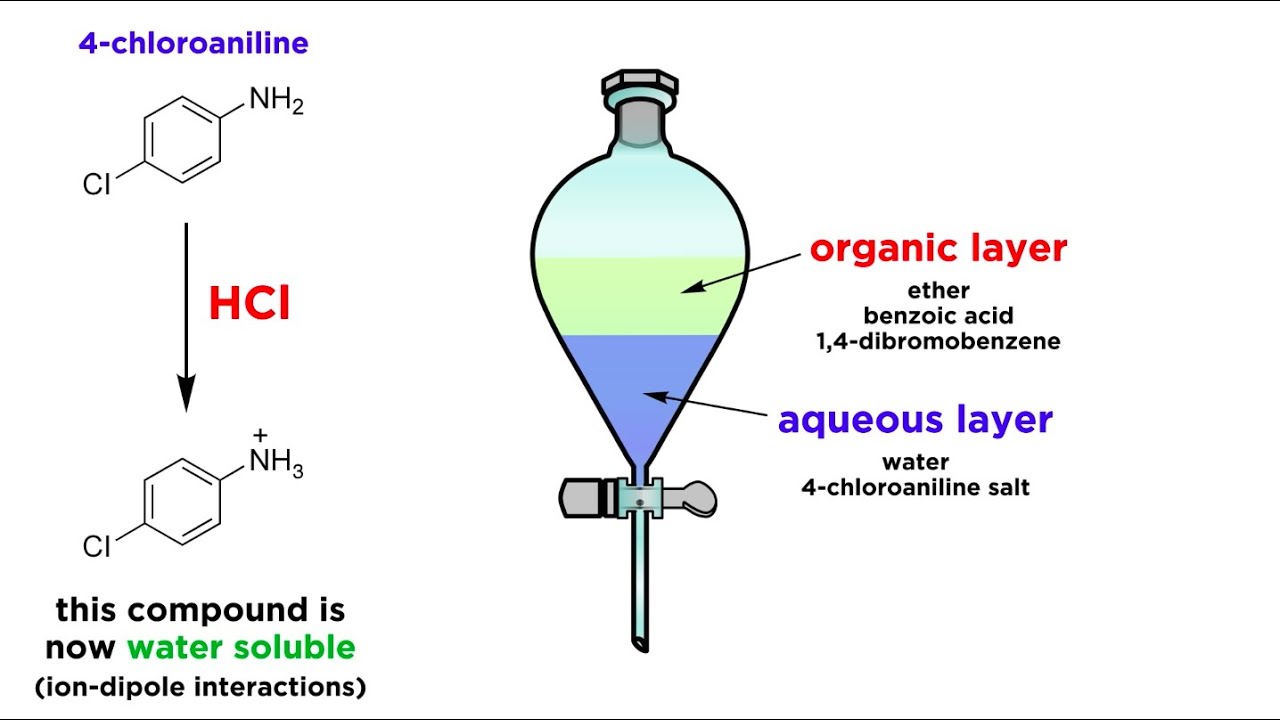
Many liquidliquid extractions are based on acidbase chemistry The liquids involved have to be immiscible in order to form two layers upon contact Since most of the extractions are performed using aqueous solutions (ie, 5 % NaOH, 5 % HCl), the miscibility of the solvent with water is a crucial point as well as the compatibility of the reagent with theCompounds Extraction Methods are widely used in various Industries for Separation of components and has wide range of applications Details of basic theories applicable to types of Extraction such as Liquid Liquid Extraction, Solid Phase Extraction, Solid Liquid Extraction and Supercritical Extraction, etc including the Types of extraction There are different types of extraction, the two main ones being liquidliquid and liquidsolid extraction In liquidliquid extraction the component you want to transfer (called the solute here and called B in the previous explanation) sits in a liquid (A) B has to be extracted into another liquid
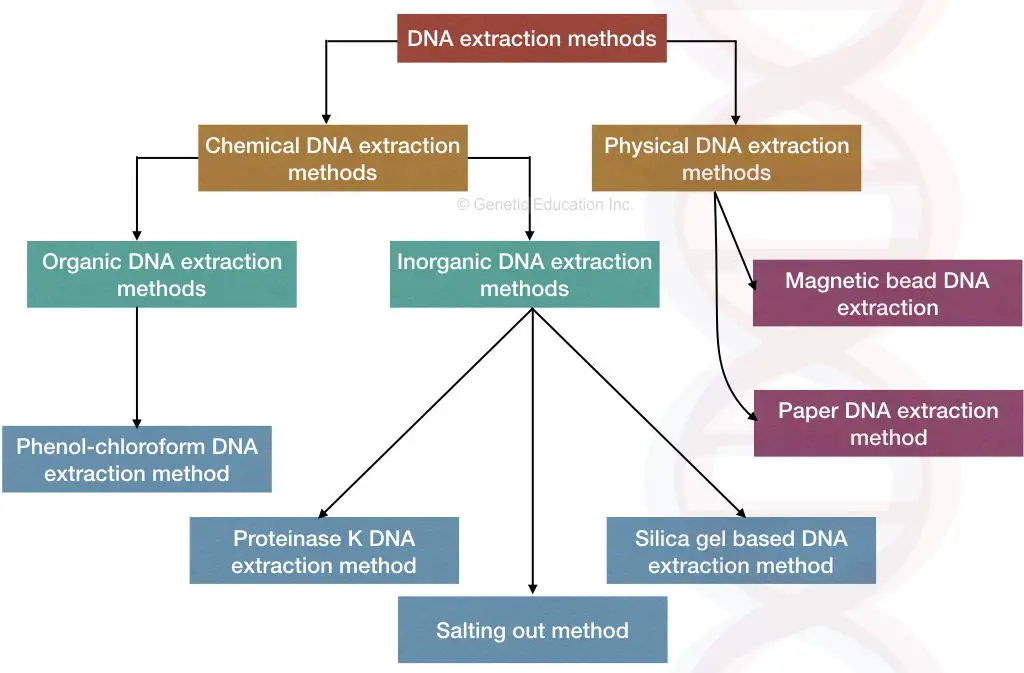



Different Types Of Dna Extraction Methods
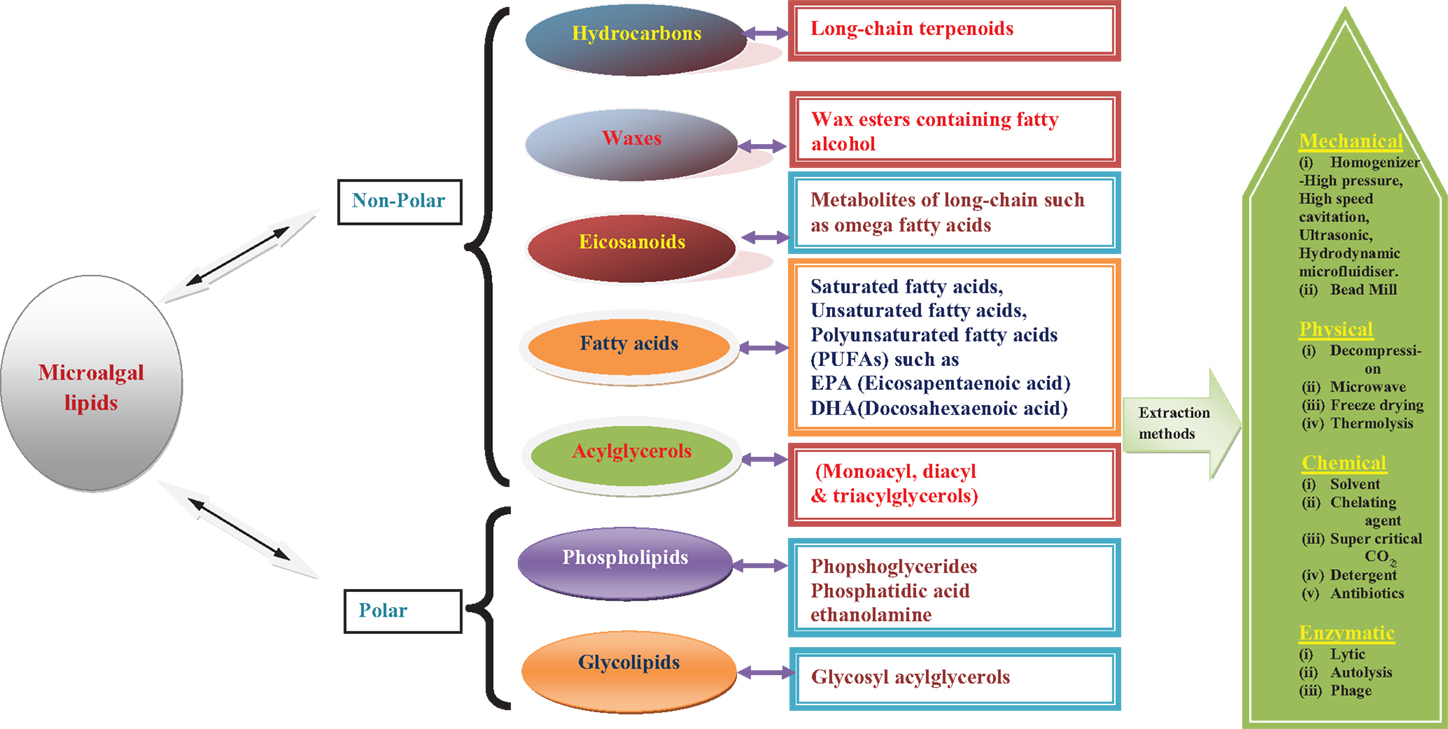



Frontiers Lipid Extraction Methods From Microalgae A Comprehensive Review Energy Research
One of the important way of classifying extractions is based on the mode of operation, gererally reffered as batch or continuousThe terms batch and continuoLearn How the Extraction of Metals from Ores is Done Metallurgy is a fascinating chapter of chemistry students like to study In this chapter, the different types of metals, their ores and different extraction processes are taughtChemistry Dictionary Definition of Extraction Extractions are a way to separate a desired substance when it is mixed with others The mixture is brought into contact with a solvent in which the substance of interest is soluble, but the other substances present are insoluble




Soxhlet Extraction An Overview Sciencedirect Topics




Extractions Video Chemical Processes Khan Academy
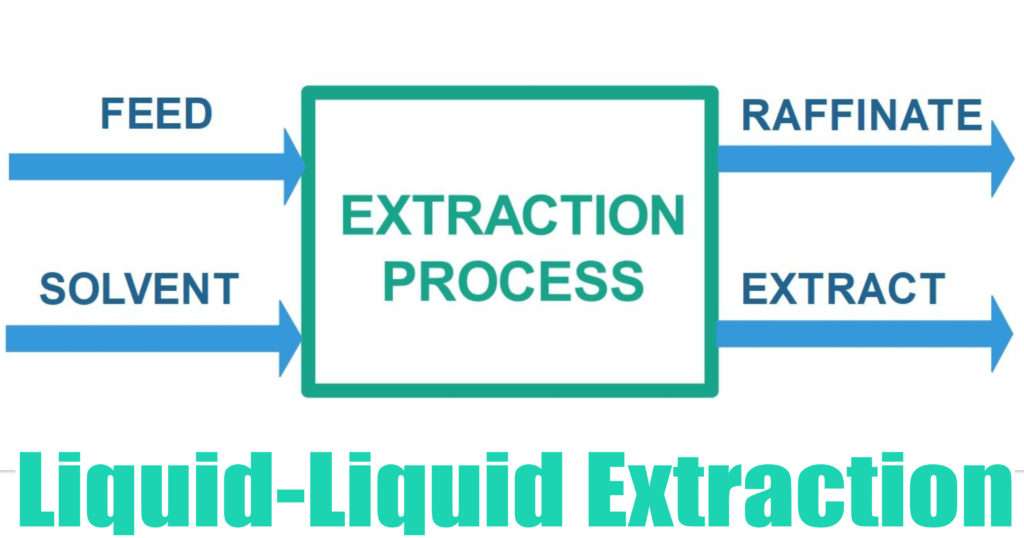
Back to EFFICIENCY FINDER FOR CHEMICAL INDUSTRY 1 OBJECTIVE The objective of extraction is to remove one constituent from a solid or liquid by means of a liquid solvent These techniques fall into two categories the first is used to dissolve soluble matter from its mixture with an insoluble solid and it's called leaching or solid extraction and the second is used to separate two miscibleLiquid liquid extraction is based on the transfer of a solute substance from one liquid phase into another liquid phase according to the solubilityExtraction becomes a very useful tool if you choose a suitable extraction solvent R You can use extraction to separate a
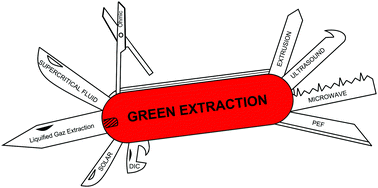



A Review Of Sustainable And Intensified Techniques For Extraction Of Food And Natural Products Green Chemistry Rsc Publishing




Green Extraction Methods For Polyphenols From Plant Matrices And Their Byproducts A Review Ameer 17 Comprehensive Reviews In Food Science And Food Safety Wiley Online Library




Advantages And Disadvantages Of The Extraction Methods Mentioned Download Table



Difference Between Distillation And Extraction Definition Technique Different Types




Liquid Liquid Extraction Chemical Engineering World
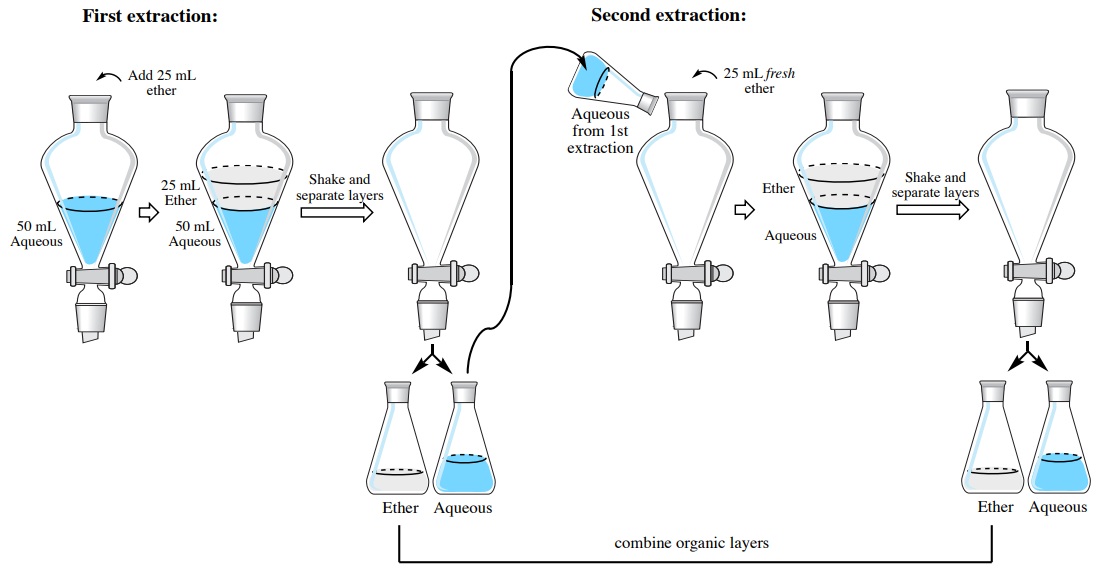



4 6 Step By Step Procedures For Extractions Chemistry Libretexts
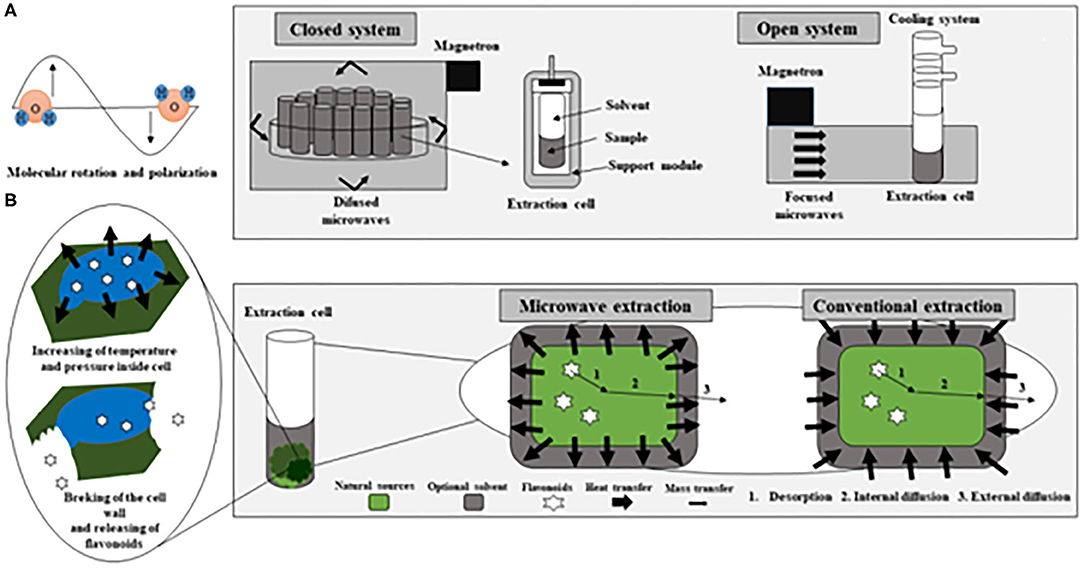



Frontiers Extraction Of Flavonoids From Natural Sources Using Modern Techniques Chemistry



Methods Of Extraction Refining And Concentration Of Fish Oil As A Source Of Omega 3 Fatty Acids
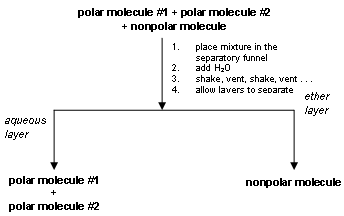



Extraction




Types Of Solvent Extraction Methods Continuous Extraction Stripping Continuity Solvent Method
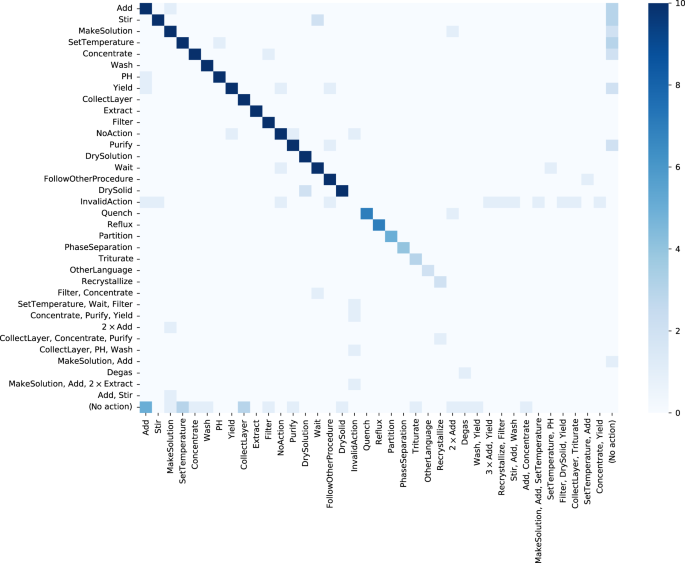



Automated Extraction Of Chemical Synthesis Actions From Experimental Procedures Nature Communications




What Is Extraction The Theory And How It S Applied To Food Food Crumbles



The Complete Guide To Hydrocarbon Extraction New In 19




Extraction




Doc Types Of Extraction Dwi Alviryansyah Academia Edu




Types Of Solvent Extraction Batch Extraction Single Double Multiple Solvent Chemistry Type
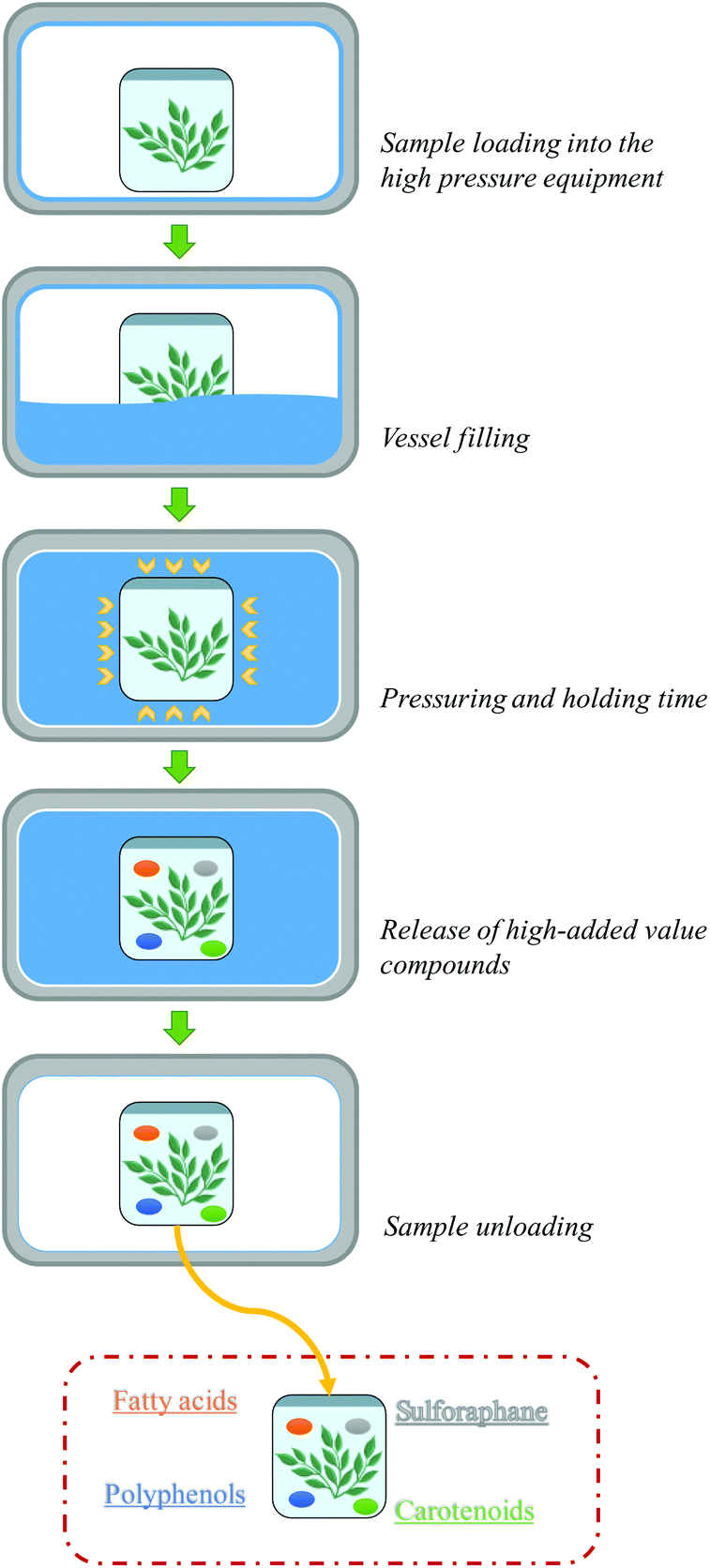



A Review Of Sustainable And Intensified Techniques For Extraction Of Food And Natural Products Green Chemistry Rsc Publishing Doi 10 1039 C9gcg
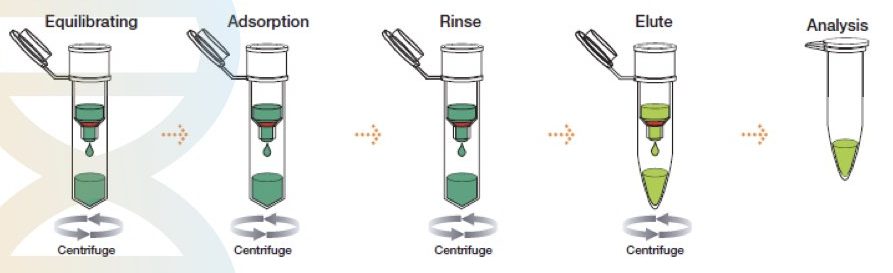



Different Types Of Dna Extraction Methods



2




Extraction




Extraction Chemistry Wikipedia
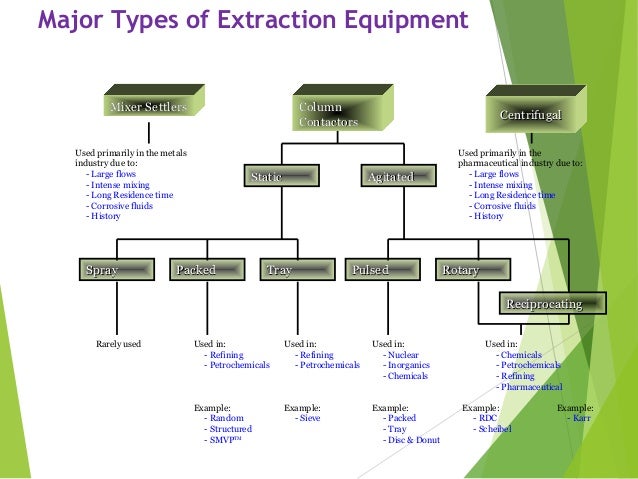



Liquid Liquid Extraction And Flocculation




Nucleic Acid Extraction Methods Biochain Institute Inc



Extraction Techniques In Sample Preparation Encyclopedia
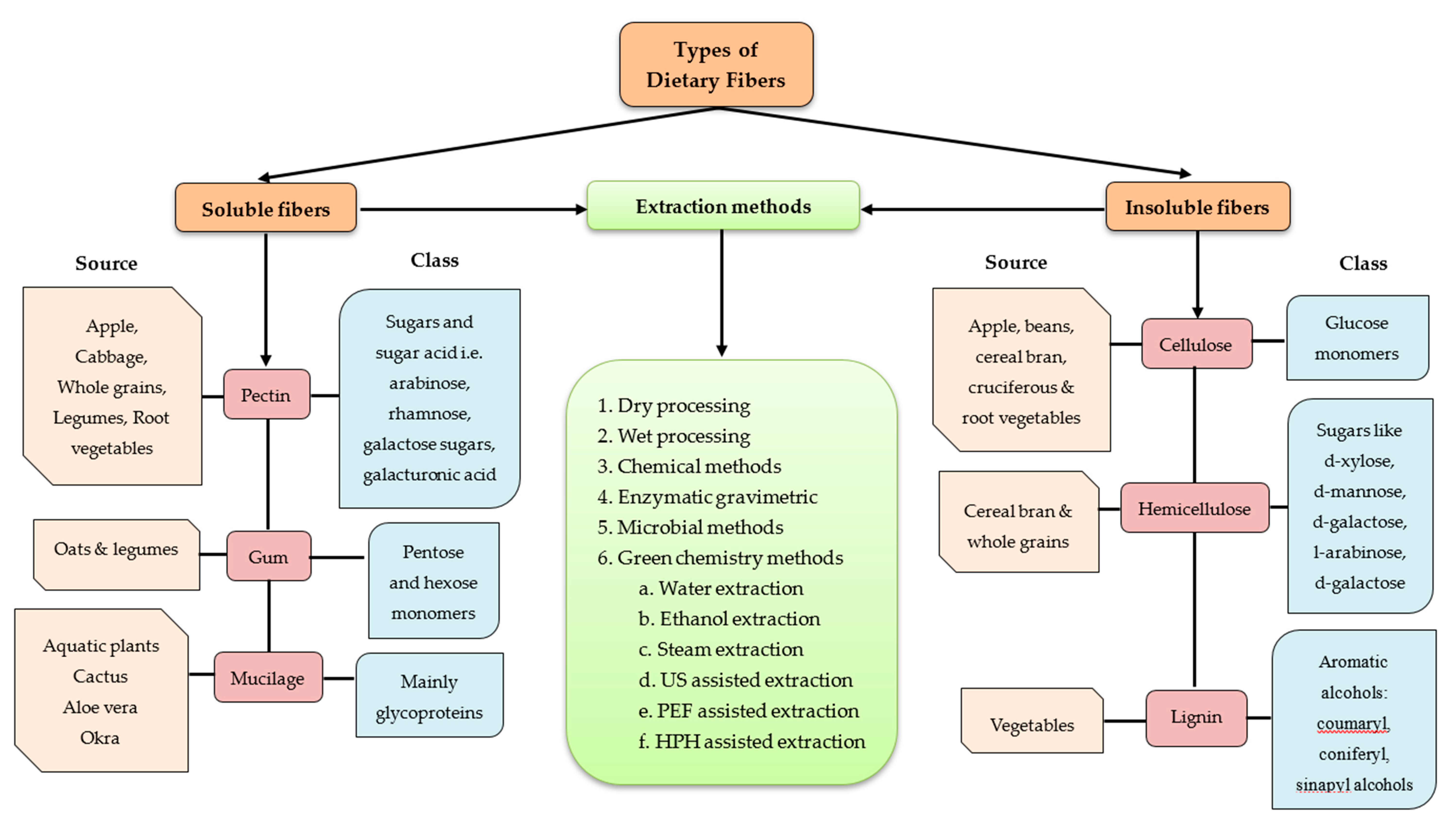



Sustainability Free Full Text Dietary Fiber From Underutilized Plant Resources A Positive Approach For Valorization Of Fruit And Vegetable Wastes Html



1



What Is The Difference Between Separation And Extraction Techniques In Chemistry Quora




Solid Phase Extraction Wikipedia




Solvent Extraction Research Proceedings On The Fifth International Conference On Solvent Extraction Chemistry 5th Icsec Held In Jerusalem Israel 16 18 September 1968 Kertes A Marcus Y Editors Amazon Com Books




A Comprehensive Guide To Essential Oil Extraction Methods




Green Alternative Methods For The Extraction Of Antioxidant Bioactive Compounds From Winery Wastes And By Products A Review Sciencedirect



Chem 211 Techniques




Ingredients Extraction By Physicochemical Methods In Food Volume 4 1st Edition
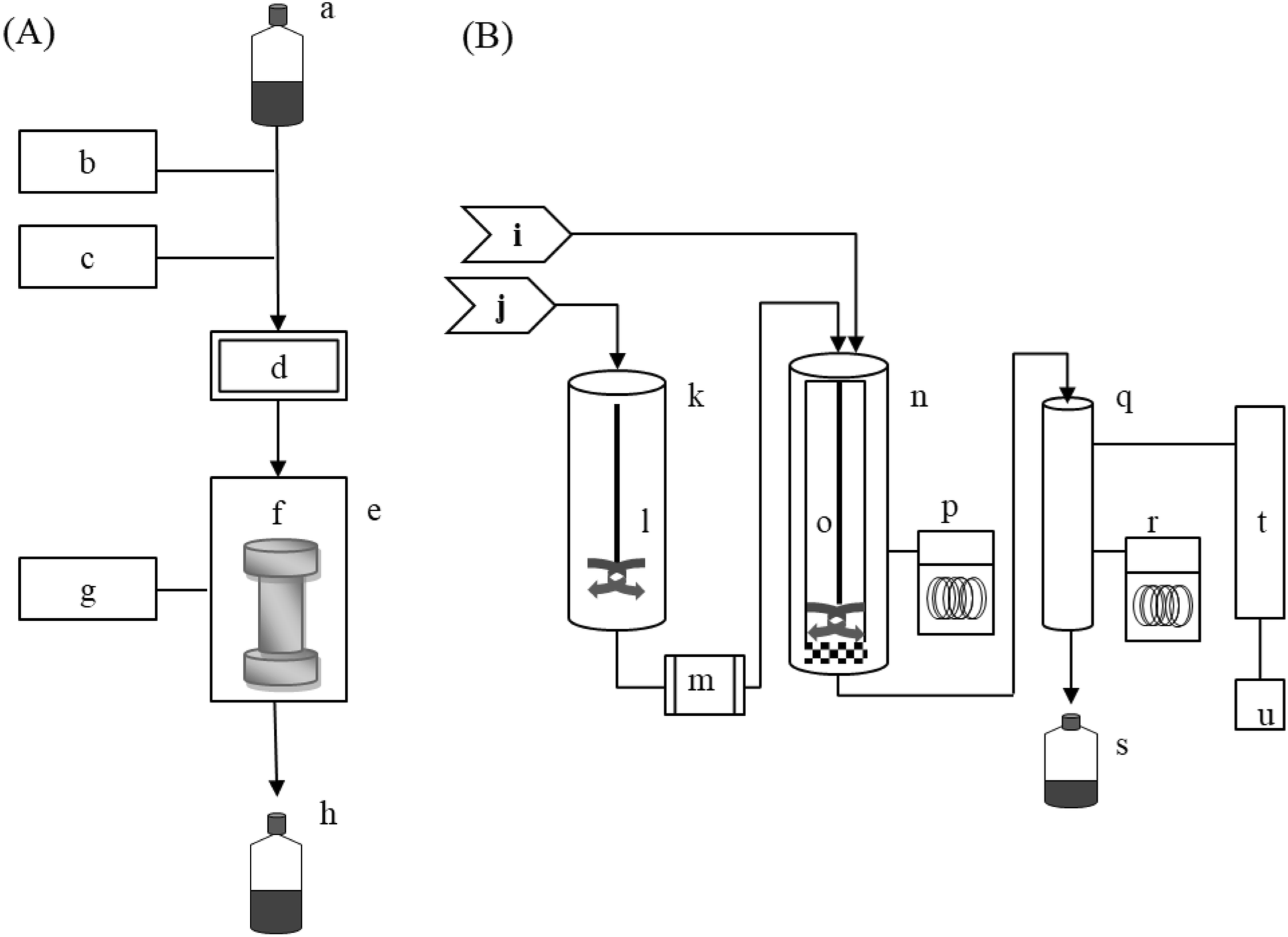



Subcritical Water Extraction Of Bioactive Compounds From Orostachys Japonicus A Berger Crassulaceae Scientific Reports




Soxhlet Extractor Percolator Boiler And Reflux Distillation Flask On Heating Element Organic Chemistry Class Pharmacy Extraction Stock Photo Picture And Royalty Free Image Image
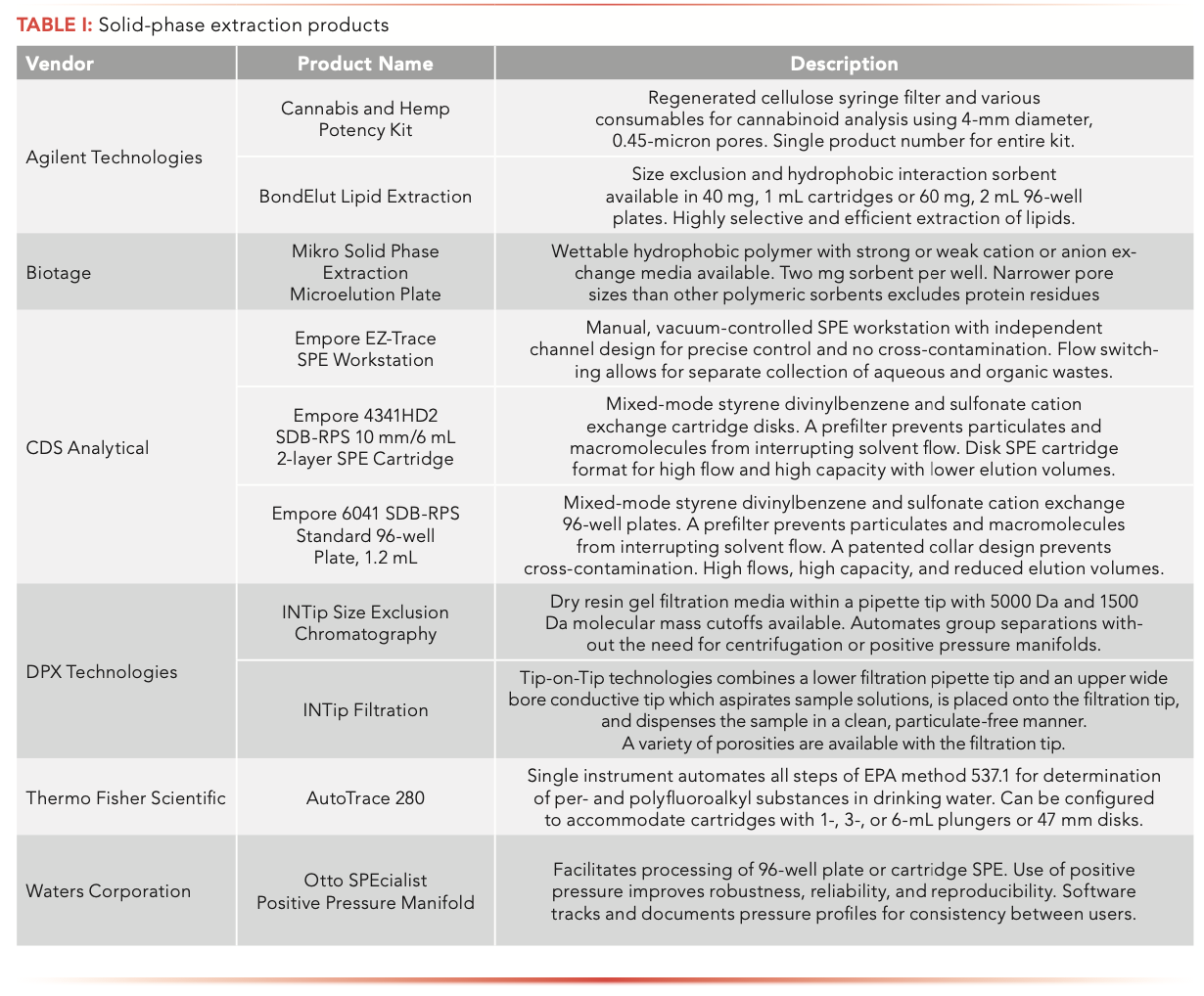



Practical Aspects Of Solvent Extraction
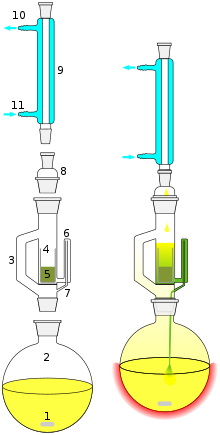



Extraction Chemistry Wikipedia



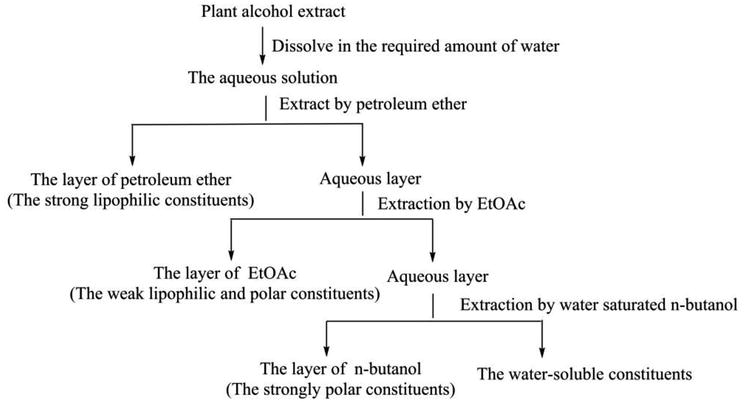
Techniques For Extraction And Isolation Of Natural Products A Comprehensive Review Chinese Medicine Full Text



4 2 Overview Of Extraction Chemistry Libretexts




Solvent Extraction 1st Year Chemistry Swap Education Portal Youtube




3 Why Is The Ph Of The Aqueous Layer Tested During Chegg Com




Plant Extraction Berkem Extraction Vegetale



Different Types Of Dna Extraction Methods




Pdf Comparison Of Chemical Extraction Methods For Determination Of Soil Potassium In Different Soil Types




5 Different Types Of Extraction And Experiments Differenttypes Net




Dna Extraction Protocol Choosing Whole Blood Dna Isolation Method



Liquid Liquid Extraction Chemical Engineering World



1




What Is Extraction The Theory And How It S Applied To Food Food Crumbles
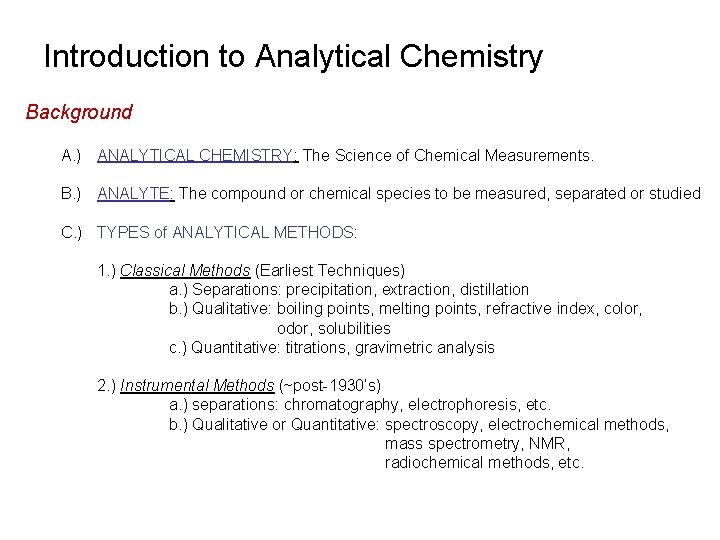



Problems In Analytical Chemistry Chem 4 Spring 15




Extraction Of Metals Cie Igcse Chemistry Revision Notes
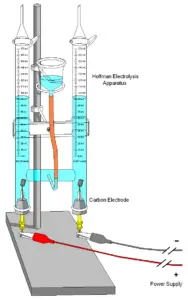



5 Different Types Of Extraction And Experiments Differenttypes Net
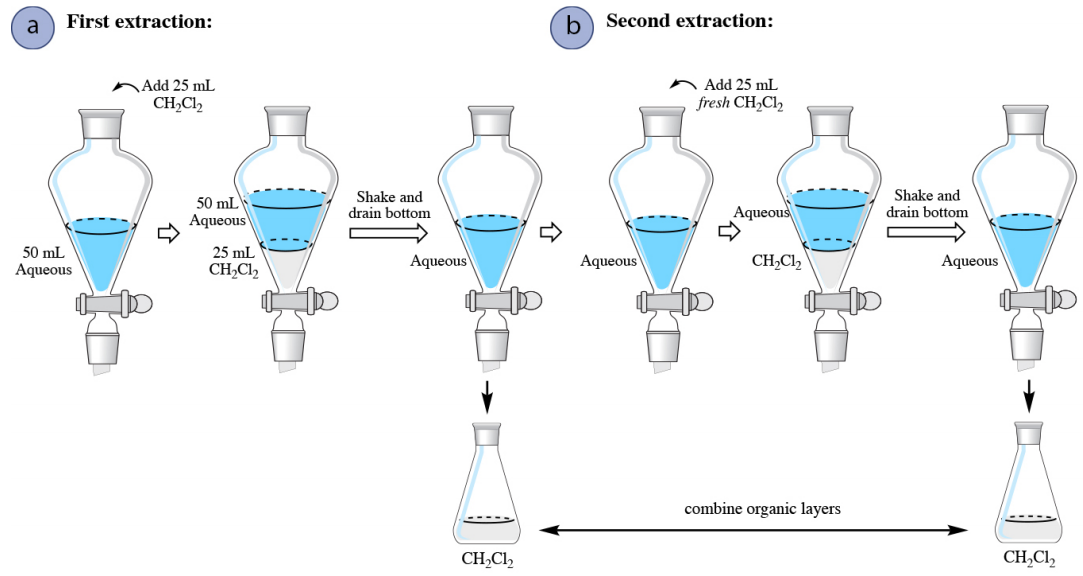



4 5 Extraction Theory Chemistry Libretexts



Extraction Chemistry Wikipedia




Plant Extraction Berkem Extraction Vegetale




Biology Free Full Text Microwave Assisted Extraction For Microalgae From Biofuels To Biorefinery Html




Extraction Liquid Liquid
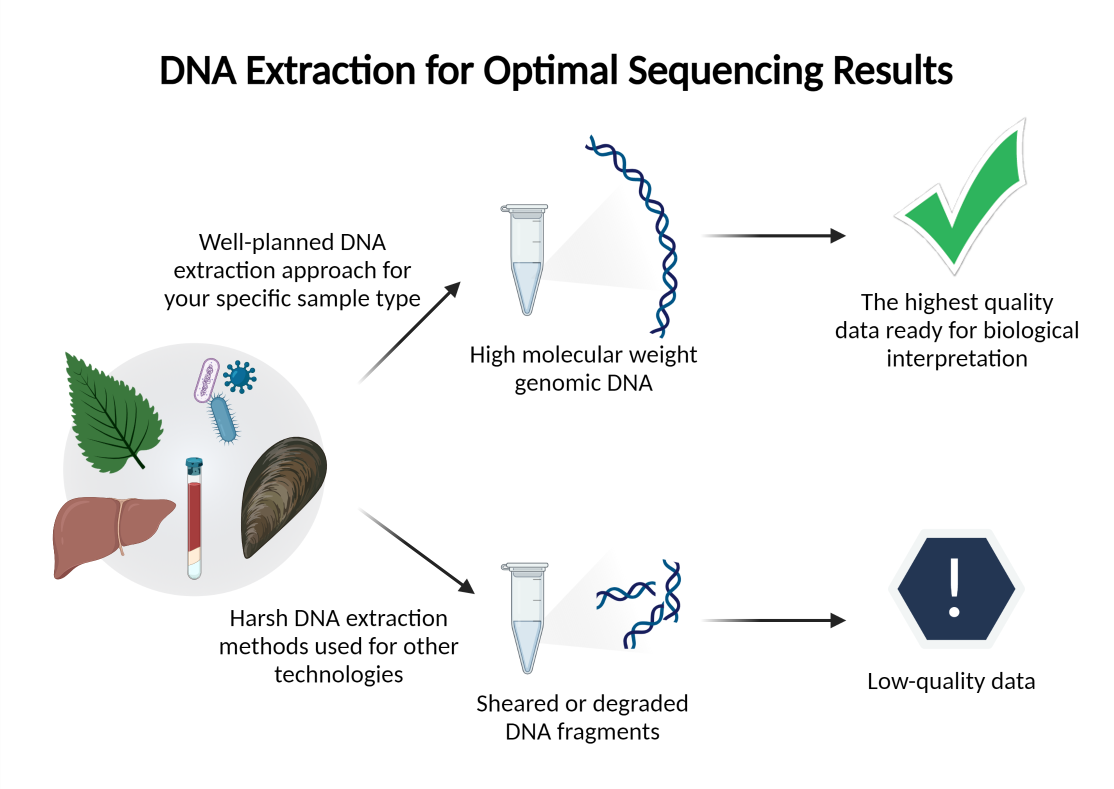



Sequencing 101 Dna Extraction Tips Kits Protocols Pacbio




A Critical Analysis Of Extraction Techniques Used For Botanicals Trends Priorities Industrial Uses And Optimization Strategies Sciencedirect




Oil Extraction An Overview Sciencedirect Topics
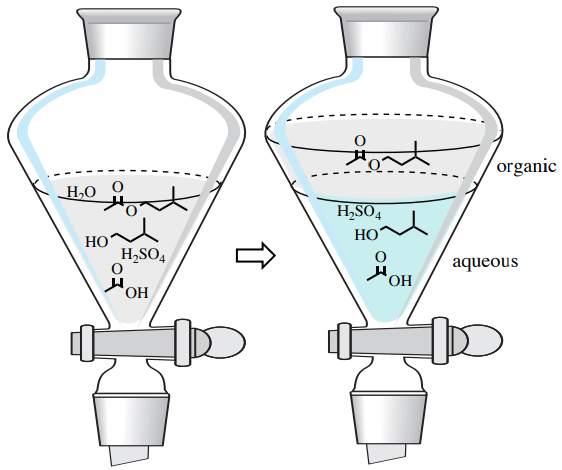



4 3 Uses Of Extraction Chemistry Libretexts




3 Main Types Of Extraction For Cbd




Types Of Extraction Process And Spagyric Extract Trust The Herb



Separation Of An Unknown Mixture




Solvent Extraction The World S Most Sophisticated Technique For Oil Extraction By Mectech Process Engineers Issuu




Solved Questions For An Organic Chemistry Lab For The Chegg Com




Solid Phase Extraction Spe Guide Waters
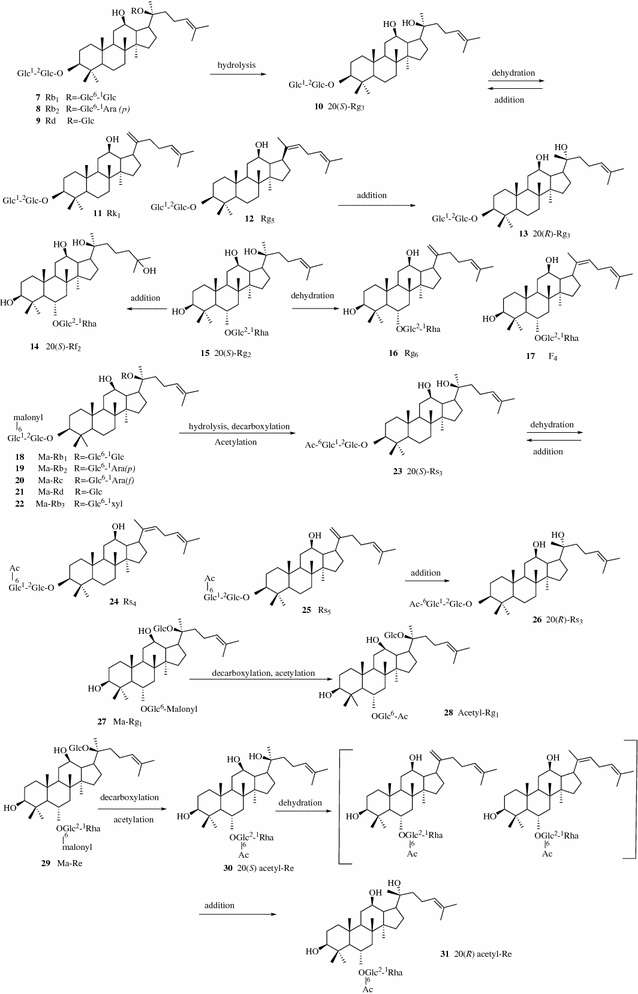



Techniques For Extraction And Isolation Of Natural Products A Comprehensive Review Chinese Medicine Full Text




Frontiers Lipid Extraction Methods From Microalgae A Comprehensive Review Energy Research




Solvent Extraction Definition Process Video Lesson Transcript Study Com



Solvent Extraction Images Stock Photos Vectors Shutterstock



1
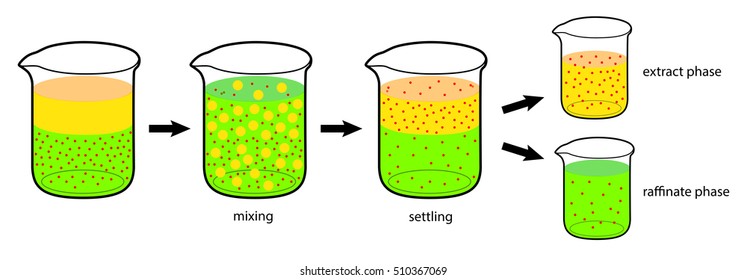



Solvent Extraction Images Stock Photos Vectors Shutterstock




Chemical Extraction Of Organic Compound From Water Solution To Organic Solvent Diagram Educational Chemistry For Kids Cartoon Style Vector Illustration Royalty Free Cliparts Vectors And Stock Illustration Image
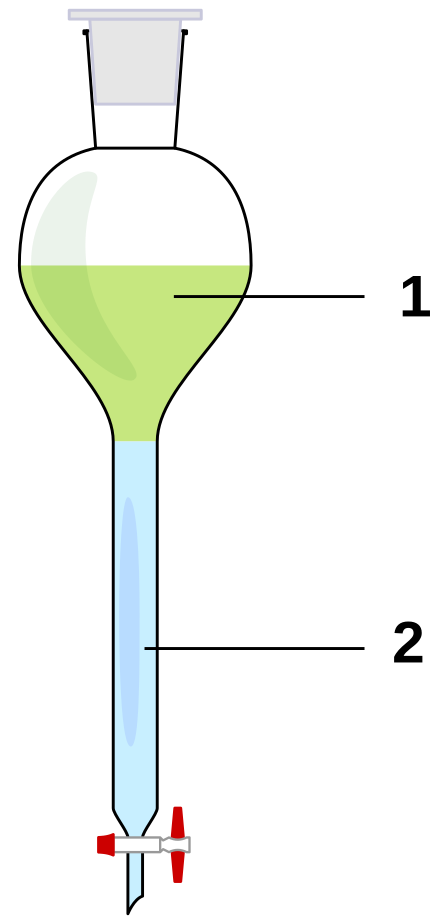



Extraction Chemistry Wikiwand




Your Guide To Ethanol Extraction In Cannabis Cannabis Business Times




Extraction Chemistry Alchetron The Free Social Encyclopedia




Extraction




Plant Solvent Extraction Method Using Ethanol 3 Steps Cole Parmer
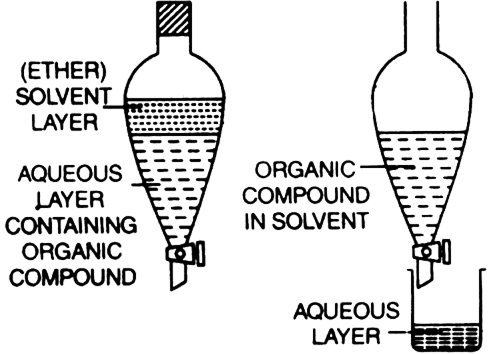



Describe Briefly The Process Of Differential Extraction From Chemistry Organic Chemistry Some Basic Principles And Techniques Class 11 Haryana Board English Medium




Solvent Extraction Or Separation Youtube




Extraction Of Caffeine From Tea Leaves Formal Report


